Draw the trend for METALLIC CHARACTER Metallic character. Ge P O d.
Atomic Radius - Overview.

. Circle the atom in each pair that. Draw the trend for ATOMIC RADIUS. It becomes smaller.
F K Br _____ Mg Na Cs _____ Os Ni Fe _____. In this video we will learn how the atomic radius of elements change as you move across the periodic table. From smallest to largest.
Periodic trends worksheet use the periodic table and your knowledge. Atomic Radius The size of an atom. F K Br _____ Os Ni Fe _____ Rank each of the following in order of DECREASING atomic radius.
What causes this trend. Identify each element as a metal metalloid or nonmetal. What trend in atomic radius do you see as you go down a groupfamily on the periodic table.
1 Rank the following elements by increasing atomic radius. Periodic Table Trends Worksheet. What causes this trend.
A Al B b S O c Br Cl d Na Al e O F f Mg Ca 6. Atomic radius is one half the distance between the nucleus of two bonding atoms. Whats people lookup in this blog.
Up to 24 cash back Periodic Trends Worksheet Atomic Radius 1. The periodic trends for atomic radius are explained using the number of electrons andor protons the number of occupied orbits the SHIELDING EFFECT and the force of attraction between the nucleus and valence electrons. Periodic Trends Practice Worksheet Briefencounters from briefencountersca Worksheet 7 trends on the periodic table topic 2 answer key.
Within a period protons are added to the nucleus as electrons are being added to the same principal energy level. A li or cs b cl or ar c ca or br d na or ne e b or be 2. A fluorine b germanium c zinc d phosphorous e lithium 3.
IONIC RADIUS For each of the following sets of ions rank them from smallest to largest ionic radius. Os Ni Fe Ni Fe Os Rank each of the following in order of DECREASING atomic radius a. Discuss the importance of Mendeleevs periodic law.
C N Al e. Students will be able to identify which atoms will have a smallerlarger atomic size and will also be able to explain w. Li C F b.
Therefore the attraction between the. Pat Ligon Created Date. Rank each of the following in order of INCREASING atomic radius.
Circle the atom in each pair that has the largest atomic radius. Gr -fCrnS - cvrCrr7 C- ui -fyn n cc- ItS fryc c aHvcc Activity on Trends on the Periodic Table i rr i M -page 4 of 4. Rank each of the following in order of INCREASING atomic radius a.
Wcpss Last modified by. Trends in the Periodic Table A discussion of the periodic trend in atomic radius. What trend in atomic radius do you see as you go across a periodrow on the periodic table.
F K Br FBrK b. Cl Br Ga Ga Br Cl b. Below is a rough sketch of the Periodic Table.
Wcpss Last modified by. Put the following elements in order from smallest to largest atomic radius. Young Brenda Created Date.
2 Group 2A Element Atomic Number Atomic Radius Be 4 111 Mg 12 160 Ca 20 197 Sr 38 215 Ba 56 217 Atomic Radius Atomic Number. Ca Rb C Rb Ca C Circle the atom in each pair that has the largest atomic radius. Arrows to show increasing periodic trends or decreasing periodic trends.
Periodic Trends Worksheet Author. What trend in atomic radius do you see as you go across a periodrow on the periodic table. There are some small exceptions such as the oxygen radius being slightly greater than the nitrogen radius.
Within a period protons are added to the nucleus as electrons are being added to the same principal energy level. The atomic radius of atoms generally decreases from left to right across a period. Within a period protons are added to the nucleus as electrons are being added to the same principal energy level.
Draw the trend for ATOMIC RADIUS. Periodic Trends Worksheet ATOMIC RADIUS 1. A summary worksheet activity of the periodic table trends including.
Carbon aluminum oxygen potassium. Oxygen carbon aluminum potassium. Rank each of the following in order of INCREASING.
The size increases when the principal quantum number increases n What is the trend in sized atomic radius within the same period going to the right and why does this occur. Rank each of the following in order of INCREASING atomic radius. The atomic radius of atoms generally decreases from left to right across a period.
Up to 24 cash back Periodic Trends Worksheet Name_____ Date_____Pd_____ Draw the trend for ATOMIC RADIUS 1. F K Br _____ Os Ni Fe _____ Rank each of the following in order of DECREASING atomic radius. Li Na K c.
Mg 2 Ca 2 Ba2 c. Sulfur oxygen neon aluminu m. Rank each of the following in order of INCREASING atomic radius a.
Defines atomic radius and explains trends in atomic radius. F K Br _____ b. Worksheet 3-3 Name Periodic Trends Period 1.
Suggest a reason for this trend based on atomic radius size and the distance and force of attraction between the nucleus and the outer electron. Periodic Trends Worksheet Author. Rank each of the following in order of DECREASING atomic radius.
ATOMIC RADIUS For each of the following sets of atoms rank the atoms from smallest to largest atomic radius. Using the data below make a bar graph of atomic radius vs. Periodic Table Trends Atomic Radius Worksheet Electronegativity decreases across a period and decreases down a group The major properties we will study are electronegativity electron affinity atomic radius and ionization energy This causes the electron to move closer to the nucleus thus increasing the.
Up to 24 cash back Periodic Trends Worksheet - Solutions. Electronegativity Electron Affinity Ionization Energy Ionic Radius Atomic Radius and Shielding Effect. The Size of an Atom.
There are some small exceptions such as the oxygen radius being slightly greater than the nitrogen radius. Trends of the Periodic Table - Atomic size RadiusThis lesson plan will teach your students about the atomic size radius trend within groups and periods of the Periodic Table. The atomic radius of atoms generally decreases from left to right across a period.
Mg 2 Si 4- S 2-b. Draw the trend for ATOMIC RADIUS. Use the Periodic Table and your knowledge of periodic trends to answer the following questions.
A The SHIELDING EFFECT. What causes this trend. Measured from the center of the nucleus to the outside of the electron cloud.
Sketch in whether the following increase or decrease going across a period left to right or going up a group. Explore fun printable activities for K-8 students covering math ELA science more. Electronegativity atomic radius electron affinity ionization energy.
1 Explaining the atomic radius trend within a group. Al Cl Ga 2. At grade 10 11 12.
The Size of an Atom Interactive. Rank each of the following in order of INCREASING atomic radius. Os Ni Fe _____ 2.
Atomic number for Group 2A and for Period 3 of the periodic table. What causes this trend. What is the trend in size atomic radius going down a group on the periodic table and why does this occur.
Periodic Trends Worksheet 2 Draw the trend for ATOMIC RADIUS Define Atomic Radius. Mg Na Cs _____ c. There are some small exceptions such as the oxygen radius being slightly greater than the nitrogen radius.
Up to 24 cash back 4. 2 Rank the following elements by increasing electronegativity. Name____Key_____ Date_____Pd_____ Draw the trend for ATOMIC RADIUS.
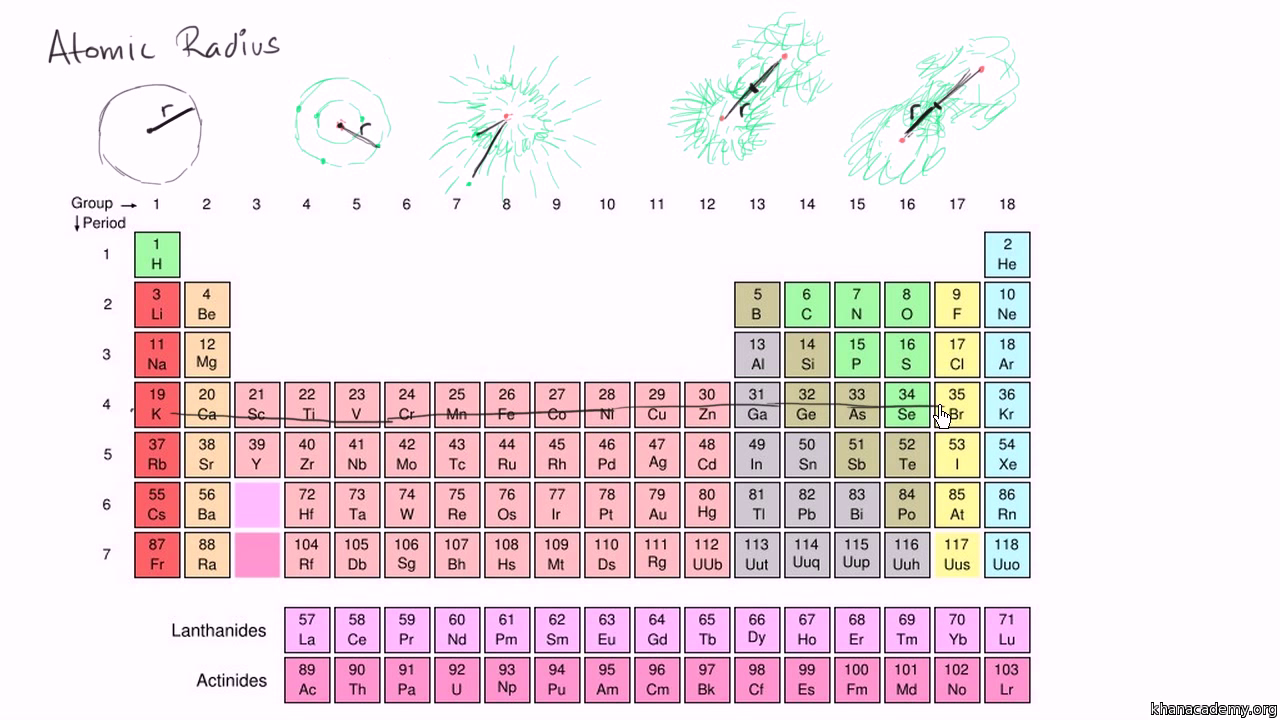
Atomic Radius Trends On Periodic Table Video Khan Academy
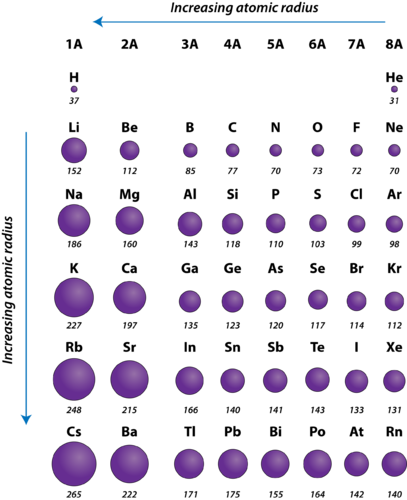
Periodic Trends Atomic Radius Chemistry For Non Majors
Atomic Radius Periodic Table Trend Chemistry Homework Worksheet Tpt

Periodic Trends Guided Inquiry Activity Chemical Education Xchange
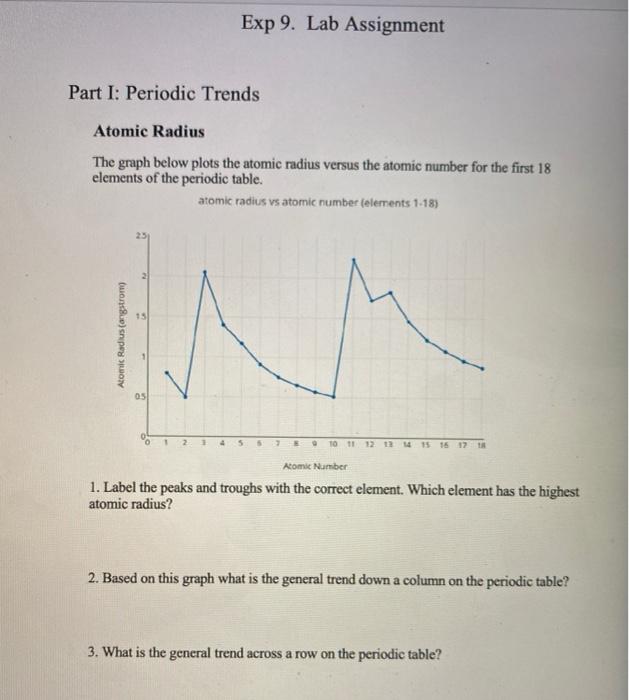
Solved Exp 9 Lab Assignment Part I Periodic Trends Atomic Chegg Com

The Periodic Table A Compilation Of Fun Chemistry Classroom Teaching Chemistry Chemistry Worksheets

Periodic Trends Question 1 Understanding The Basis Of The Trends A

Periodic Trends Webquest And Graphing Ps1 1 Graphing Ionization Energy Webquest

0 comments
Post a Comment